Complete notes on Hydrocarbons i.e, Alkanes, Alkenes & Alkynes for IIT JEE, NEET by Chem Infusion
- Chem Infusion
- Dec 4, 2019
- 3 min read
HYDROCARBONS
Classification
Alkanes
Alkenes
Alkynes
Aromatic Hydrocarbons
TIPS TO REMEMBER
Organic compounds composed of only carbon and hydrogen are called hydrocarbons.

Alkanes
The saturated hydrocarbons are represented by the general formula CnH2n+2.
General properties :
The normal alkanes are colourless gases (C1 to C4), colourless liquids (C5 to C17) and from C18 onwards colourless solids.
As branching increases, melting and boiling points decrease. The boiling point increases
steadily with increase in molecular mass.
Density of alkanes also increases with size of the molecule.
They are generally insoluble in polar solvents like water but soluble in non-polar solvents like ether, chloroform, etc.
Methods of Preparations of Alkanes :
1. By Hydrogenation of unsaturated hydrocarbons (Sabatier & Senderen's reactions)
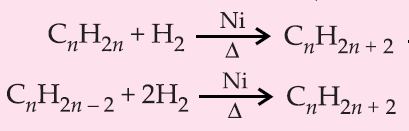
2. Wurtz Reaction

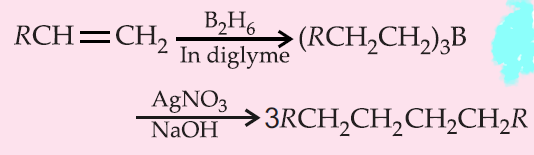
3. By hydroboration of alkenes :
4. Corey-House synthesis :

5. Kolbe's electrolysis :

6. From Grignard's Reagent :

7. From Carbonyl compounds (Clemmensen Reduction) :

Chemical Properties of Alkanes:
1. Halogenation:
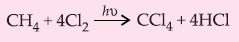
Mechanism of halogenation : The mechanism is believed to involve the following steps :
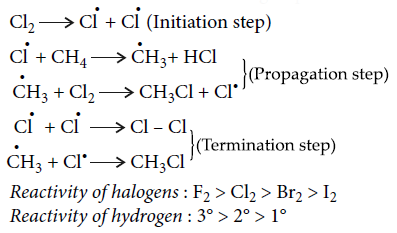
2. Nitration:

3. Sulphonation:

4. Complete Combustion:
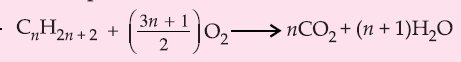
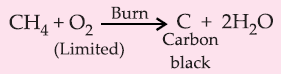
5. Incomplete Combustion:
6. Catalytic Oxidation :

7. Isomerisation :
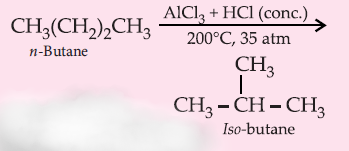
8. Aromatisation :
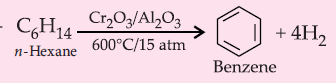
9. Pyrolysis or Cracking :

Click to know What is pyrolysis?
This video is not our original content, we found this well explained video from to show our students.
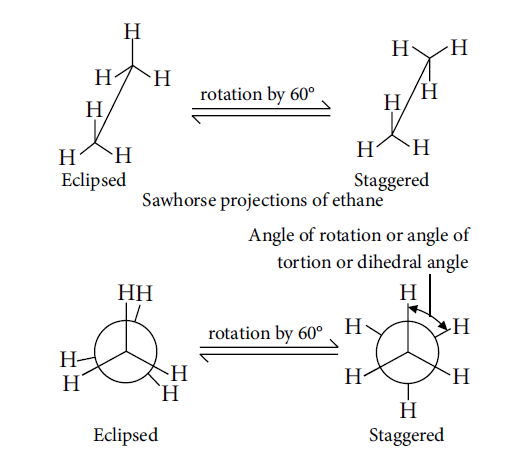
Conformations of Ethane (Sawhorse and Newman
Projections)
Staggered conformation: The hydrogen atoms attached to two carbons are far apart and
experience minimum repulsion.
Eclipsed conformation: The hydrogen atoms attached to two carbons are as close together as possible and experience maximum repulsion.
Gauche or Skew form: A rotation of 60° converts a staggered conformation into an eclipsed conformation, or vice-versa. Rotation between 0° to 60° generates one of the many other arrangements in between staggered and eclipsed forms. These arrangements are called gauche or skew form.
Order of stability: Staggered > Skew or Gauche > Eclipsed
Points to be remembered :
Methane cannot be prepared by Kolbe’s electrolytic method.
Ethane is prepared in the laboratory by electrolysis of conc. aqueous solution of CH3COOK.
The electrolysis of HCOONa gives H2 gas at both cathodes as well as the anode.
During chlorination, the relative ease of abstraction of H atom is 1: 3.8: 5 for primary, secondary and tertiary hydrogen respectively.
In bromination, the ratio becomes 1: 82: 1600 for 1°, 2°, and 3° H atoms. The yield of alkanes produced can be accordingly predicted.
Watch this video, very nicely explained the Chemical properties of carbon, Combustion.
Some Question for you to Practice
Q.1 A gaseous hydrocarbon gives upon combustion 0.72 g of water and 3.08 g of CO2. The empirical formula of the hydrocarbon is
(a) C7H8 (b) C2H4
(c) C3H4 (d) C6H5
(JEE Main 2013)
Q.2 Which branched chain isomer of the hydrocarbon with molecular mass 72 u gives only one isomer of monosubstituted alkyl halide?
(a) Neopentane (b) Isohexane
(c) Neohexane (d) Tertiary butyl chloride
(JEE Main 2012)
Write down your answer in the comment box, let's see who can do this correctly.
Alkenes
Organic compounds containing C = C are known as alkenes. Alkenes with two double
bonds are known as dienes.

Methods of preparation:
By dehydration of alcohols :

Saytzeff rule: If a single starting compound can yield two or more isomers then more substituted alkene is formed in greater amount.
By dehydrohalogenation of alkyl halides :

Partial hydrogenation of alkynes :
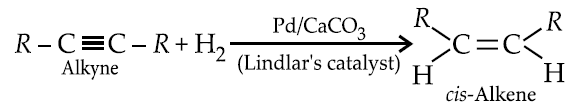
Watch the video to know in details how the Birch reduction and Lindlar's catalysts are working to give trans and Isomers respectively.
Disclaimer: This video is not made by Chem Infusion, We found it from youtube. If you love this video then do subscribe to him on youtube.
Birch reduction :
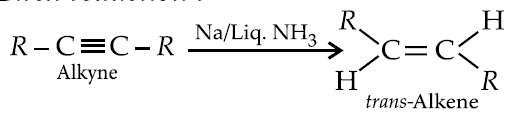
TIPS
This is a very important part of Organic Chemistry, you are gonna face it various times. You have to remember the reagents and have to know how they affect the stereochemistry.
My recommendation for students is to make a separate small notebook for Reagents itself.
Electrolysis of salt of dicarboxylic acids :

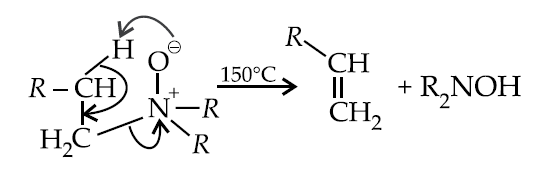
Hofmann elimination :
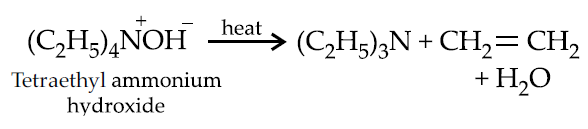
Wittig reaction :
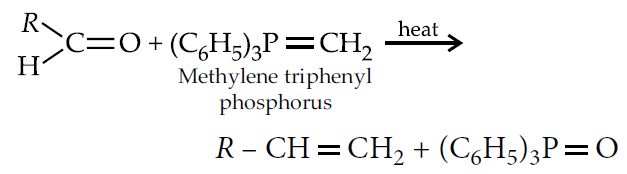
Cope reaction :

Properties :
All alkenes are colourless and odourless (except ethene). Ethene has a pleasant odour.
C1 - C3 (gases), C4 - C16 (liquids), C17 onwards solids.
Boiling point, melting point and specific densities increase with the increase in molecular mass in homologous series.
Mechanism of electrophilic addition :
Step 1: The reagent ionises

Step 2 :

Step 3 :
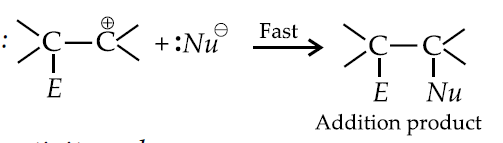
Reactivity order :
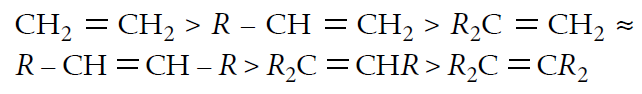
Markovnikov’s rule: The negative part of unsymmetrical reagent adds to less hydrogenated
carbon atom of double bond.
Peroxide effect: Addition of HBr in the presence of peroxide gives products opposite to
Markovnikov’s rule.

Oxymercuration-demercuration:
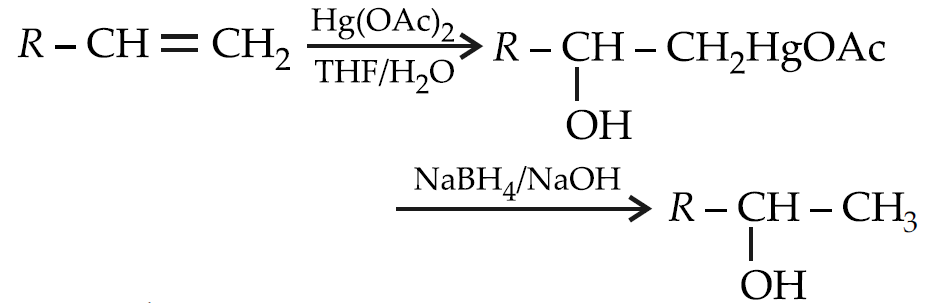
Ozonolysis:
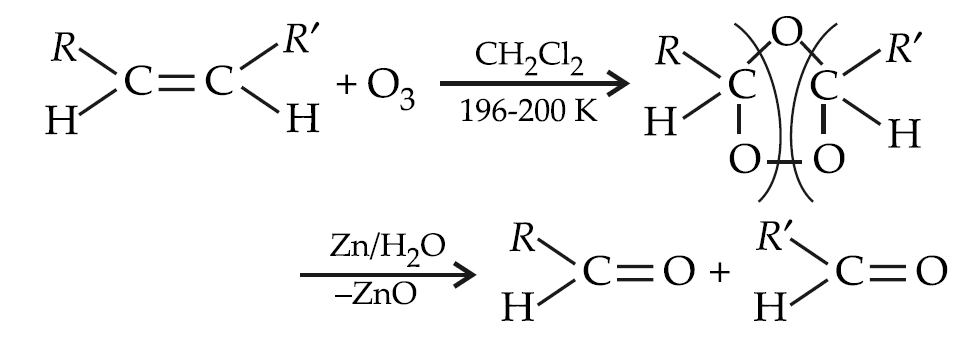
Polymerisation:

Oxidation: Hot alkaline KMnO4 or K2Cr2O7 as oxidising agent.

Points to be Remembered:
In case of dialkyl derivatives of ethylene which exist as geometrical isomers, the trans isomer is more stable than the cis isomer. This is because of lesser crowding in trans-isomers.
With alkaline KMnO4, =CH2 part of alkene is oxidised to CO2 and H2O & =CRR′ part is oxidised to RCOR′.
N-Bromosuccinimide (NBS) is used for the bromination of alkenes at the allylic position.
RhCl(Ph3P)3 is Wilkinson’s catalyst and used for the hydrogenation of alkenes.
In an another blog, we will discuss about Alkynes and Aromatic Hydrocarbons.
Do you want me to cover all organic topics?
let me know in the comment box.



Comments